
Chair of Solid-State and Quantum Chemistry
Welcome to the Chair of Solid-State and Quantum Chemistry at RWTH Aachen University, Europe's largest Technical University dating back to the year 1870. The following pages are intended to offer you a short overview of our research and teaching activities in the fields of solid-state and quantum chemistry. If you are interested in these exciting chemical disciplines, you have come to the right place. Stay with us! Don't go away!
HOT: Metavalent Bonding Demystified
Phase-change memory materials have unusual properties and important applications, and recent efforts to find improved materials have focused on their bonding mechanisms. “Metavalent bonding” or “metavalency,” intermediate between “metallic” and “covalent” bonding and comprising single-electron bonds, has been proposed as a fundamentally new mechanism that is relevant both here and for halide perovskite materials. It is shown, however, that PCMs, which violate the octet rule, have two types of covalent bond: two-center, two-electron (2c-2e) bonds, and electron-rich, multicenter bonds (3c-4e bonds, hyperbonds) involving lone-pair electrons. The latter have bond orders less than one and are examples of the century-old concept of “partial” bonds.
DOI:10.1002/adma.202300836
HOT: Crystal Structure of Carbonic Acid
Ubiquitous carbonic acid, a key molecule in biochemistry, geochemistry, and also extraterrestrial chemistry, is known from a plethora of physicochemical studies. Its crystal structure has now been determined from neutron-diffraction data on a deuterated sample in a specially built hybrid clamped cell. At 1.85 GPa, carbonic acid crystallizes in the monoclinic space group P21/c, Z = 4, with one symmetry-inequivalent anti-anti shaped D2CO3 molecule forming dimers, as previously predicted. Quantum chemistry evidences π bonding within the molecular core, very strong hydrogen bonding between the molecules, and a massive influence of the crystal field on all bonds; phonon calculations emphasize the locality of the vibrations, being rather insensitive to the extended structure.
DOI:10.3390/inorganics10090132
Solid-State Chemistry
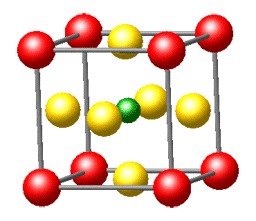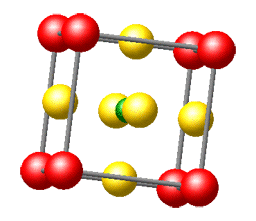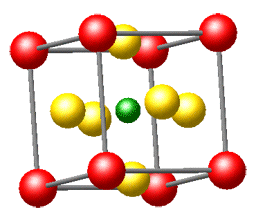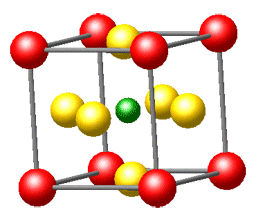
Here's the message: We honestly believe that solid-state chemistry is one of the most exciting chemical disciplines. This fundamental brand of the chemical sciences brings us into contact with a large part of the "real world" surrounding us, and a creative solid-state chemist is in true command of the entire periodic table when he or she decides to make new compounds with often unforeseeable but exciting physical properties. Solid-state chemistry is truly interdisciplinary and borders with solid-state physics, crystallography, quantum theory, metal science, and inorganic chemistry, to name but a few; also, it is one of the rock-solid platforms on which the increasingly popular fields of nanoscience and nanomaterials may be built.
Some of the breathtaking technological advances of the 20th, and also the early 21st century, would have been totally impossible without the fundamental research originating within solid-state chemistry, for example cleverly designed insulators such as dielectric ceramics for data transmission, novel ionic conductors for energy storage in hand-held electrical devices, magnetic intermetallics and oxides for data storage applications, advanced nitrides for electro-optical and diverse mechanical purposes, and also superconductors for energy transport and communication applications. In addition, there is also curiosity-driven research in solid-state chemistry, touching upon chemical systems you probably have never heard of. Interested? Read more about our research to become addicted...
Teaching
 No research today? The teaching section is intended to inform chemistry (and other) students about the various chemistry courses offered by this chair at the Institute of Inorganic Chemistry. No research today? The teaching section is intended to inform chemistry (and other) students about the various chemistry courses offered by this chair at the Institute of Inorganic Chemistry.
|
Computational Chemistry

Computational chemistry is an ingenious, non-experimental way to solve chemical problems by means of sheer computation on the basis of hard-core numerical methods (which are typically quantum-chemical in nature), and this approach has become an increasingly important part of the chemical sciences. Our group specializes in the quantum chemistry of solids (well, that's not too surprising) and we surely know how to solve Schrödinger's equation for periodic systems. In fact, there has been huge progress in properly describing the whole universe of solid-state materials (insulators, semiconductors, metals, and intermetallic compounds) by electronic-structure theory; in addition, predictive conclusions are now in our own hands.
While the numerical methods of ours include very different quantum-chemical tools, their varying levels of accuracy and speed are due to differences in the atomic potentials and the choice of the basis sets involved. The latter may either be totally delocalized (plane waves) or localized (atomic-like), adapted to the valence electrons only (pseudopotentials) or to all the electrons. In order to understand structures and compositions, the results of electronic-structure theory are investigated in terms of further quantum-chemical bonding analyses. There are also cases where one would like to know more about the dynamical behavior of the various atoms, and then the time evolution of their spatial coordinates (that is, their "trajectories") must be calculated as a function of the macroscopic temperature, for example by molecular-dynamics approaches. Go to our research section to learn more about theory and computation. It's fun!
Location
 Although the history of Aachen reaches back to Roman times about 2,000 (and more) years ago, RWTH Aachen University is relatively young for European (not American) standards since it was founded at the end of the 19th century, at the peak of the industrial revolution. Today, RWTH Aachen University is Europe's largest technical university with very famous engineering schools, and its national as well as international reputation also goes back, in part, to its chemistry division. Find out more about our location and our laboratories. If you come from outer space, you may prefer to have a look at our institute from the sky using Google Earth (see top of page). Although the history of Aachen reaches back to Roman times about 2,000 (and more) years ago, RWTH Aachen University is relatively young for European (not American) standards since it was founded at the end of the 19th century, at the peak of the industrial revolution. Today, RWTH Aachen University is Europe's largest technical university with very famous engineering schools, and its national as well as international reputation also goes back, in part, to its chemistry division. Find out more about our location and our laboratories. If you come from outer space, you may prefer to have a look at our institute from the sky using Google Earth (see top of page).
|
|

 Impressum (Imprint) |
Administration |
Print
Impressum (Imprint) |
Administration |
Print



